- Effect of Interface on the Properties of Polyamide 6/Carbon Nanotube Nanocomposites Prepared by In-situ Anionic Ring-opening Polymerization
Jin Hong Min*, Mongyoung Huh**, Seok Il Yun*†
* Department of Chemical Engineering and Materials Science, Sangmyung University, Seoul 03016, Korea
*† Department of Chemical Engineering and Materials Science, Sangmyung University, Seoul 03016, Korea
** Affiliation Korea Institute of Carbon Convergence Technology, Jeonju 54853, Korea
Multiwalled carbon nanotubes (MWCNTs) are covalently
functionalized with isocyanates by directly reacting commercial hydroxyl
functionalized MWCNTs with excess 4,4'-methylenebis (phenyl isocyanate) (MDI)
and hexamethylene diiosocyanate (HDI). HDI-modified MWCNTs results in a higher
surface isocyanate density than MDI-modified MWCNTs. Anionic ring-opening
polymerization of e-caprolactam is conducted
using a sodium caprolactam initiator in combination with a di-functional
hexamethylene-1,6-dicarbamoylcaprolactam activator in the presence of
isocyanate functionalized MWCNTs. This polymerization proceeds in a highly
efficient manner at relatively low reaction temperature (150oC) and short reaction times (10
min). During the polymerization, the isocyanate functionalized MWCNTs act not
only as reinforcing fillers but also as second activators. Nanocomposites with
HDI modified MWCNTs exhibit higher reinforcement and faster isothermal
crystallization than MDI modified MWCNTs. The results show that PA6 chains grow
more effectively from HDI modified MWCNT surface than from MDI modified MWCNT
surface, resulting in stronger interaction between PA6 and MWCNTs.
Keywords: PA6, MWCNT, Isocyanate, Nanocomposite, Anionic ring-opening polymerization
Polyamide
6 (PA6) is one of the most widely used engineering plastics due to its superior
mechanical and thermal properties, oil resistance, low cost and ease of
synthesis [1-3]. These characteristics have usually been utilized to
produce filler reinforced PA6 composites in many high-end fields [4,5]. The high melting
temperature and viscosity of thermoplastics limits their growth in polymer
composites, mainly due to expensive and time-consuming manufacturing processes
such as autoclaves or hot presses. Significant reduction in matrix viscosity is
important for cost effective production of thermoplastic composites with
optimum impregnation of the reinforcements by the matrix. This can be achieved
by the reactive processing (or monomer casting) techniques, where the
thermoplastic matrices are synthesized in situ, through polymerization of
low-viscosity monomers or oligomers in the presence of the reinforcements [6-10]. The most commonly
used polymerization types is the ring-opening polymerization of e-caprolactam (ECL). In this mechanism, ring-shaped monomer
molecules are opened and transformed into high molecular weight PA6 without
liberation of by-products by means of an activator and a catalyst. The process
is carried in a way that the ECL polymerization and PA6 crystallization occur
simultaneously at temperatures 40-60oC lower than the melting point
of the resulting PA6 (220oC). This significantly reduces the overall
production cycle time and increases the energy efficiency of the process.
Despite CNTs having desirable physical properties, the full potential of
employing carbon nanotubes (CNTs) as reinforcements has been severely limited
because of the poor dispersion and poor interfacial bonding of CNTs to monomer
or polymer matrices. These problems largely stem from the smooth surface of
CNTs, which is chemically inert and incompatible with most solvents and
polymers [11-13]. Recently, the functionalization of CNTs with PA6
by using the grafting-from strategy has been adopted to improve the dispersion
and interfacial bonding of CNTs in PA6 matrix [14,15]. Utilizing
di-isocyanate as a surface modifier for hydroxyl functionalized CNT and
subsequent CNT surface initiated anionic polymerization of ECL have been a
facile route to PA6 functionalization of CNTs [14,15]. In the present
study, we used two different modifiers, 4,4'-methylenebis (phenyl isocyanate)
(MDI) and hexamethylene diiosocyanate (HDI) to incorporate isocyanate
functionalities onto the surface of MWCNTs. Anionic ring-opening polymerization
of ECL was conducted in the presence of MDI and HDI modified MWCNTs by using a
sodium caprolactam (C10) initiator in combination with a di-functional
hexamethylene-1,6-dicarbamoylcaprolactam (C20) activator. In this case, the PA6
chain grows from the MWCNT surface, while C10/C20 initiate polymerize in the bulk
ECL melt as shown in Fig.
1. To date, no direct comparison between MDI and HDI for preparing
isocyanate functionalized CNTs has been reported in the literature. In this
study, compared to unmodified CNTs, the isocyanate functionalized CNTs,
especially HDI modified CNTS, more effectively reinforced the nanocomposites
and accelerate PA6 isothermal crystallization
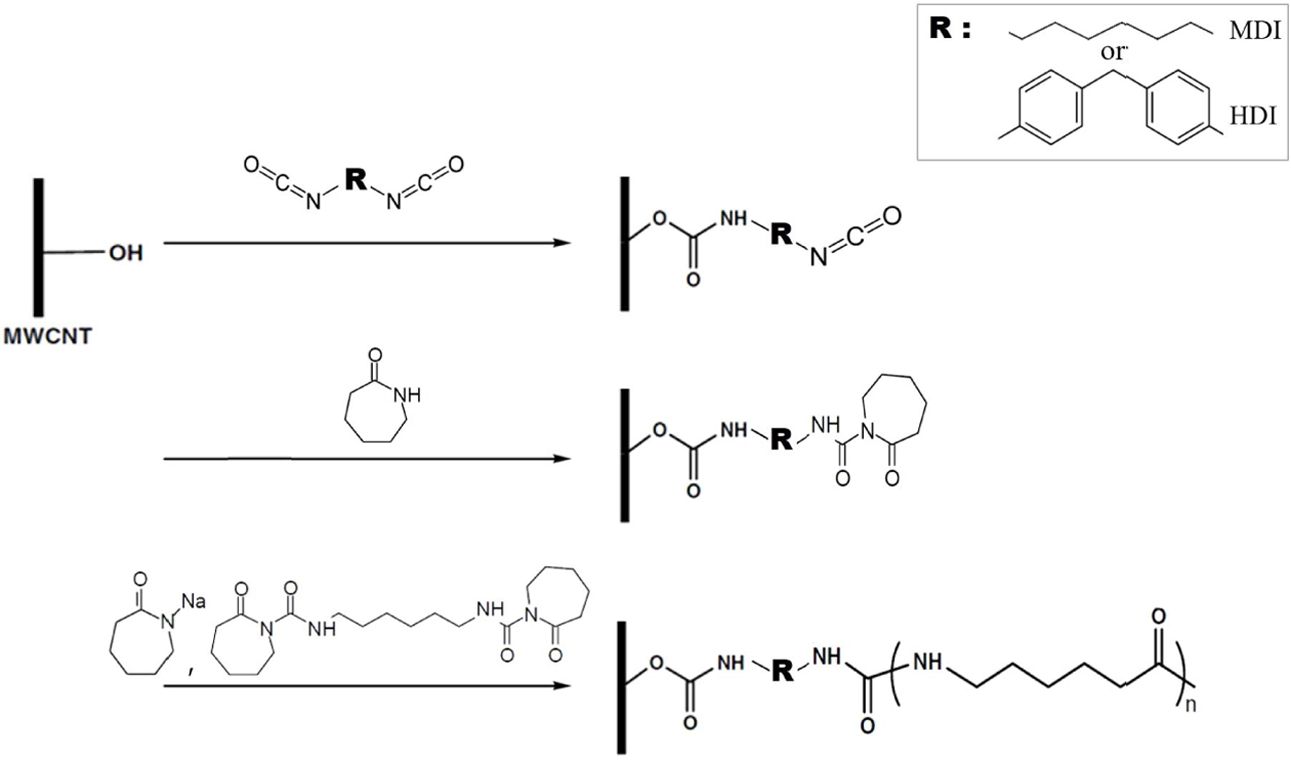
|
Fig. 1 Synthesis of isocyanate functionalized MWCNTs and subsequent PA6 chain growth from functionalized MWCNTs |
2.1
Materials
The
ECL used was an anionic polymerization grade (AP-Nylon¢ç grade) supplied by
Bruggemann Chemical (Heilbronn, Germany) as it has a low moisture content
(<100 ppm). The MDI, HDI, dibutyltin dilaurate (DBDL) and anhydrous
tetrahydrofuran (THF) were purchased from Sigma-Aldrich (US). A C20 as an
activator and a C10 initiator were purchased from Brueggemann Chemical. MWNTs-OH
(3.06 wt% OH) was purchased from US Research Nanomaterials, Inc (purity >
95%, diameter 10-20 nm, length 10-30 mm).
2.2
Surface modification of hydroxyl functionalized MWCNTs
The
CNT-OH (2 g) in anhydrous THF (100 mL) was sonicated for 20 min to obtain a
homogeneous suspension of CNT-OH, and was then put into a 100 mL three-necked
round-bottom flask. After a mechanical stirring under nitrogen (N2),
the mixture was heated to 50oC, followed by addition of 500 mol% MDI
(or HDI) relative to CNT-OH and 1.25 mL DBDL catalyst. The reaction was
maintained at 50oC for 1 h and filtered and dried.
2.3
In-situ synthesis of PA6/MWNTs Nanocomposite
ECL
monomers (100 g) were put into a three-necked flask and heated to 80°C under a
nitrogen atmosphere. After the monomer was melted, MDI (or HDI) modified MWCNTs
(0-1 wt%) were added. The mixed melt was stirred and sonicated for another 20
min. The C10 (2.5 wt%) and C20 (1.67 wt%) were added to the mixed melt then
immediately cast into a mold at 150°C and reacted for 10 min; thus, the product
of PA6 nanocomposite was obtained.
2.4
Characterization
Nanocomposite
samples were cut into thin flakes and weighed. The samples were refluxed for 24
h to remove unreacted ECL monomer in hot distilled water (95oC).
After drying, samples were weighed again. The sample weight difference was used
as 100 × (weight after refluxing / weight before refluxing) to obtain a degree
of conversion (wt%). Fourier transform infrared (FT-IR) spectra were obtained
by use of a Bruker/Tensor27 spectrometer (Germany). The spectrum of samples on
the ATR plate was collected at a 4 cm-1 resolution with 2 min
intervals by co-adding 16 scans. The isothermal crystallization of PA6 and PA6
nanocomposites was studied by differential scanning calorimetric (DSC) analysis
with a modulated DSC (Q200, TA instruments, US). The samples of about 10 mg
were heated quickly from ambient temperature to 250oC under nitrogen
atmosphere, and then held for 5 min to eliminate the effect of the previous
thermal history. Then the samples were quenched to isothermal crystallization
temperature (188, 190, and 192oC), and the isothermal
crystallization was carried out at these temperatures
The
MWCNT-OH material was reacted with MDI and HDI to produce isocyanate
functionalized MWCNTs (Fig. 1).
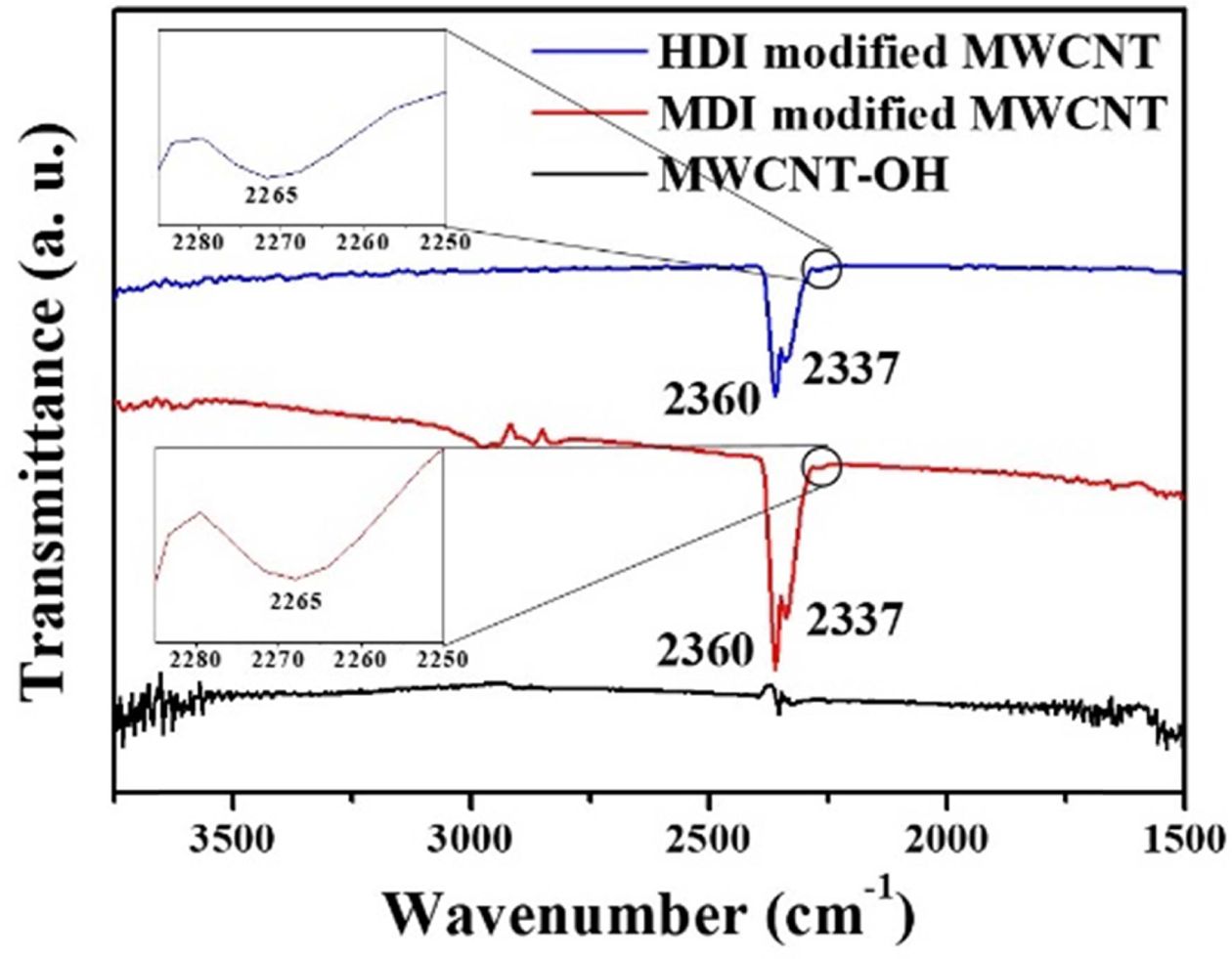
Fig.
2 shows the FTIR spectra of MWCNT-OH, isocyanate functionalized MWCNTs by
MDI and HDI. Unlike the MWCNT-OH, addition of excess TDI and MDI to the
MWCNT-OH, resulted in the appearance of clearly discernable bands at 2360 and
2337 cm-1 corresponding to C=O bond and asymmetric stretching of the
appended terminal isocyanate groups, respectively [16]. The typical band
at 2265 for isocyanate group was also observed [14,15]. The results
indicate that the isocyanates were successfully incorporated into the surface
of MWCNT-OH using the coupling agents, HDI and MDI. To gain a more quantitative
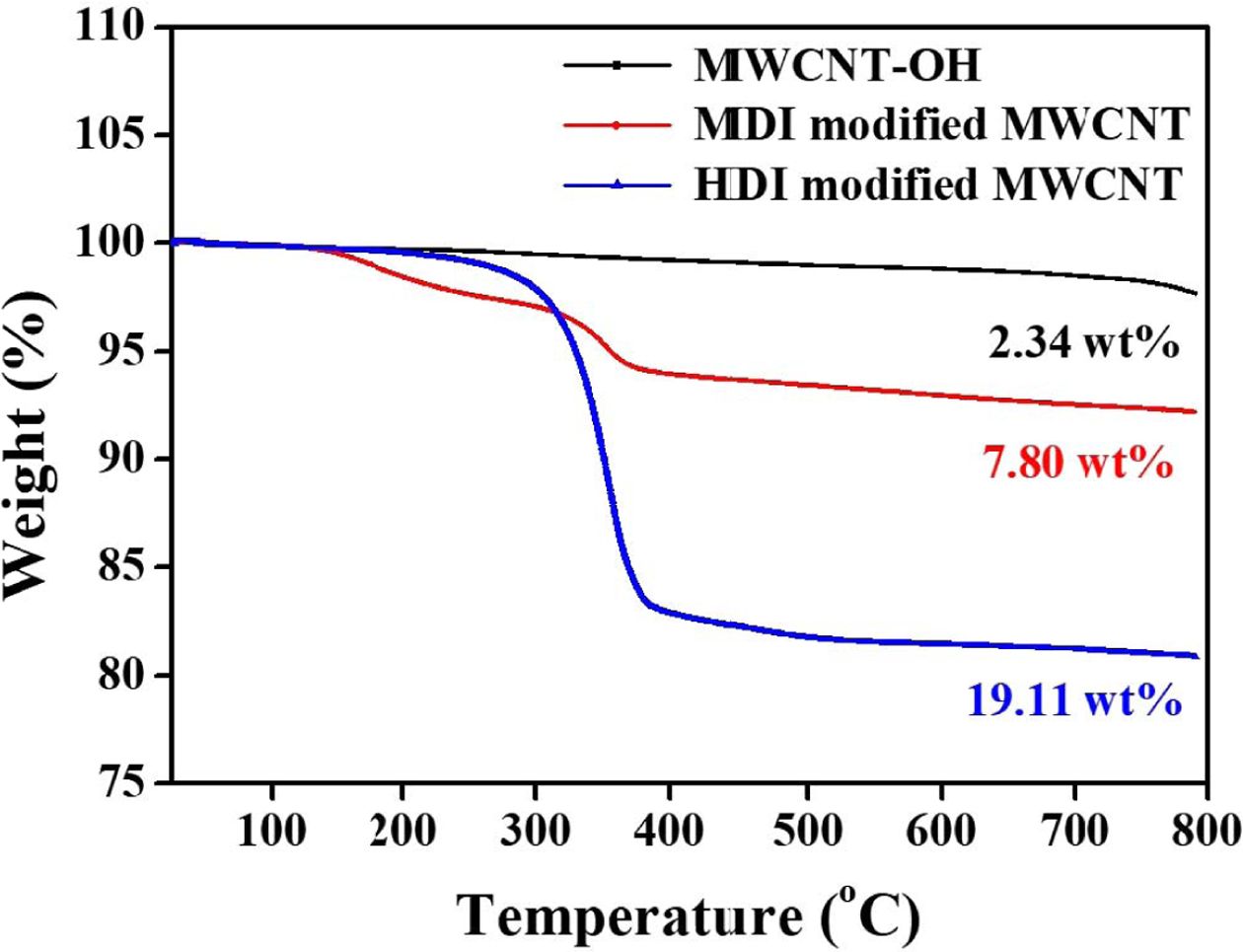
picture of the extent of nanotube functionalization, TGA analysis was performed
on the surface functionalized MWCNTs as depicted in Fig. 3. For comparison,
the TGA plot of MWCNT-OH showed a gradual mass loss of around 2.34 wt% as the
temperature reaches 800oC. There is a distinct difference in mass
loss region between 300 and 400oC for different isocyanate functionalized
MWCNTs. HDI modified MWCNTs showed much greater loss than MDI modified MWNTs.
The mass loss was found to be 7.80 and 19.11 wt%, for the MDI and HDI modified
MWCNT, respectively. Considering that the starting nanotubes have a hydroxyl
group concentration of 3.06 wt%, the TGA results indicate that about 55.4% of
the available hydroxyl groups reacted with HDI despite the poor solubility of
MWCNTs in THF. However, for MDI modified MWNTs, only a 12.% of available OH
groups reacted with MDI.
In
the presence of isocyanate functionalized MWCNTs, anionic ring-opening
polymerization of ECL was conducted by using a C10 initiator in combination
with a C20 activator. Isocyanate groups of MDI and HDI modified MWCNTs could
serve as activators of ECL during the in situ polymerization. In this case,
Polymerization occurs from the MWCT surfaces and in bulk ECL melts by C10,
simultaneously. Modified or unmodified MWCNTs were found to be well dispersed
in the molten ECL by visual inspection. The molten ECL and final composite
containing MWCNTs showed no difference with respect to CNT dispersion by CNT
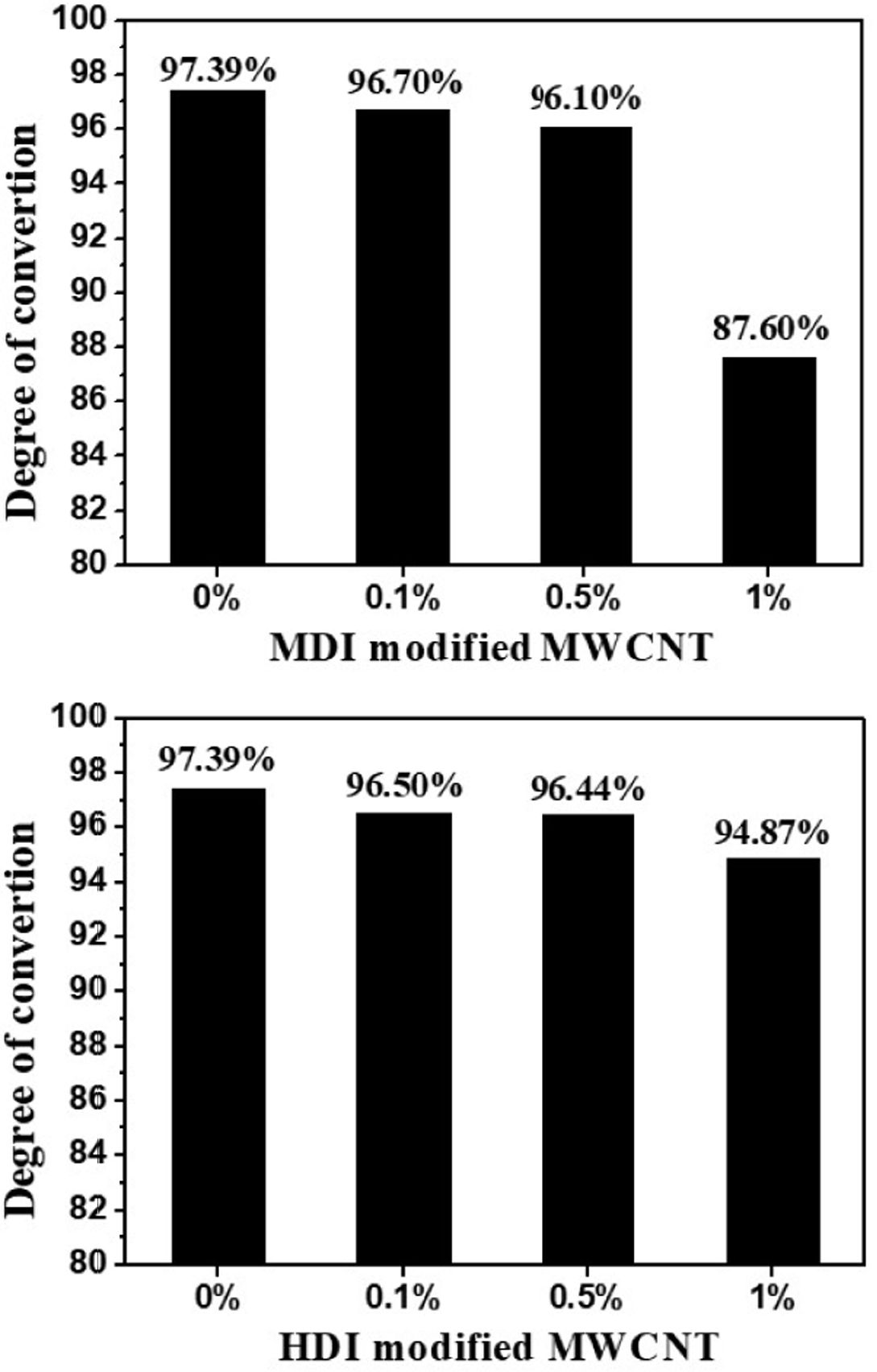
surface modification [17]. The effects of the MWCNT concentration on the degree of
conversion are presented in Fig. 4. The final degree of conversion showed the downward
trend with increasing MWCNT concentration. According to the TGA results, compared to
HDI modified MWCNTs, MDI modified MWCNTs contain more unreacted OH
functionalities that can terminate the anionic ring-opening polymerization,
resulting in the greater reduction in conversion for nanocomposites.
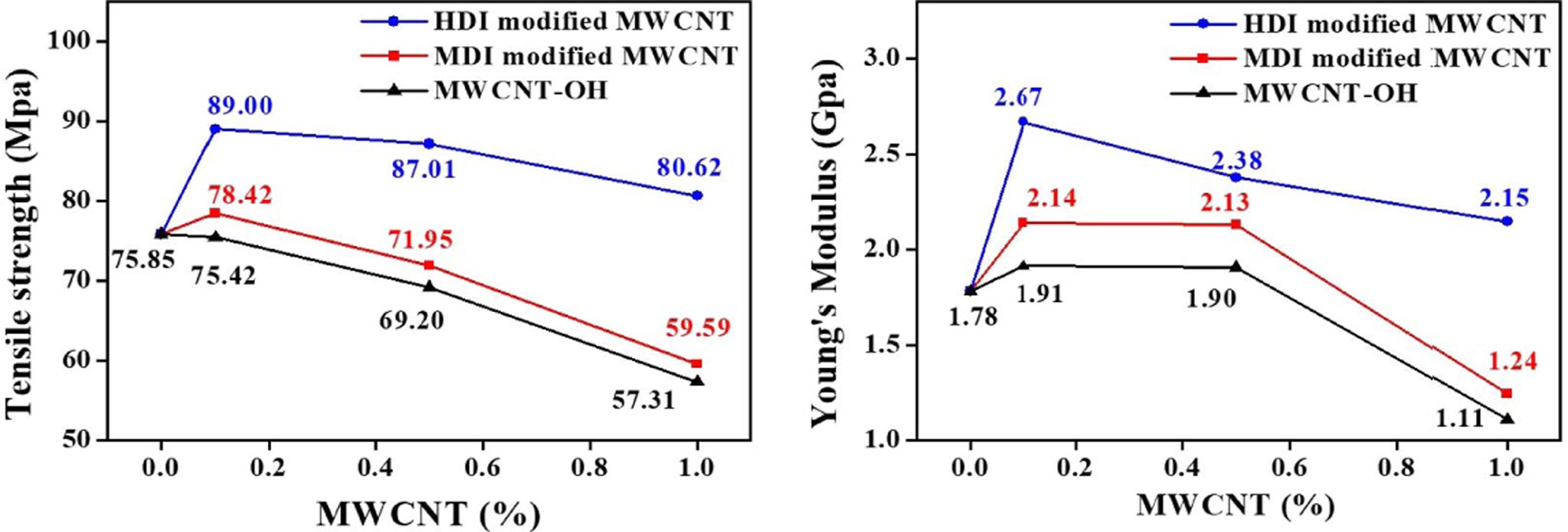
The
tensile test results of neat PA6 and PA6/MWCNTs nanocomposites with different
CNT loadings are summarized in Fig. 5. Nanocomposites with HDI-modified CNTs showed higher
mechanical improvement than MDI-modified CNTs and hydroxyl CNTs. The results
clearly displayed that higher isocyanate density on the CNT surface led to
higher PA6 grafting density during the polymerization. High PA6 grafting
density of CNT resulted in stronger interactions and better compatibility
between PA6 and MWCNT which are responsible for higher mechanical reinforcement
of nanocomposite compared to lower isocyanate density on the CNT surface (MDI
modified MWCNT).
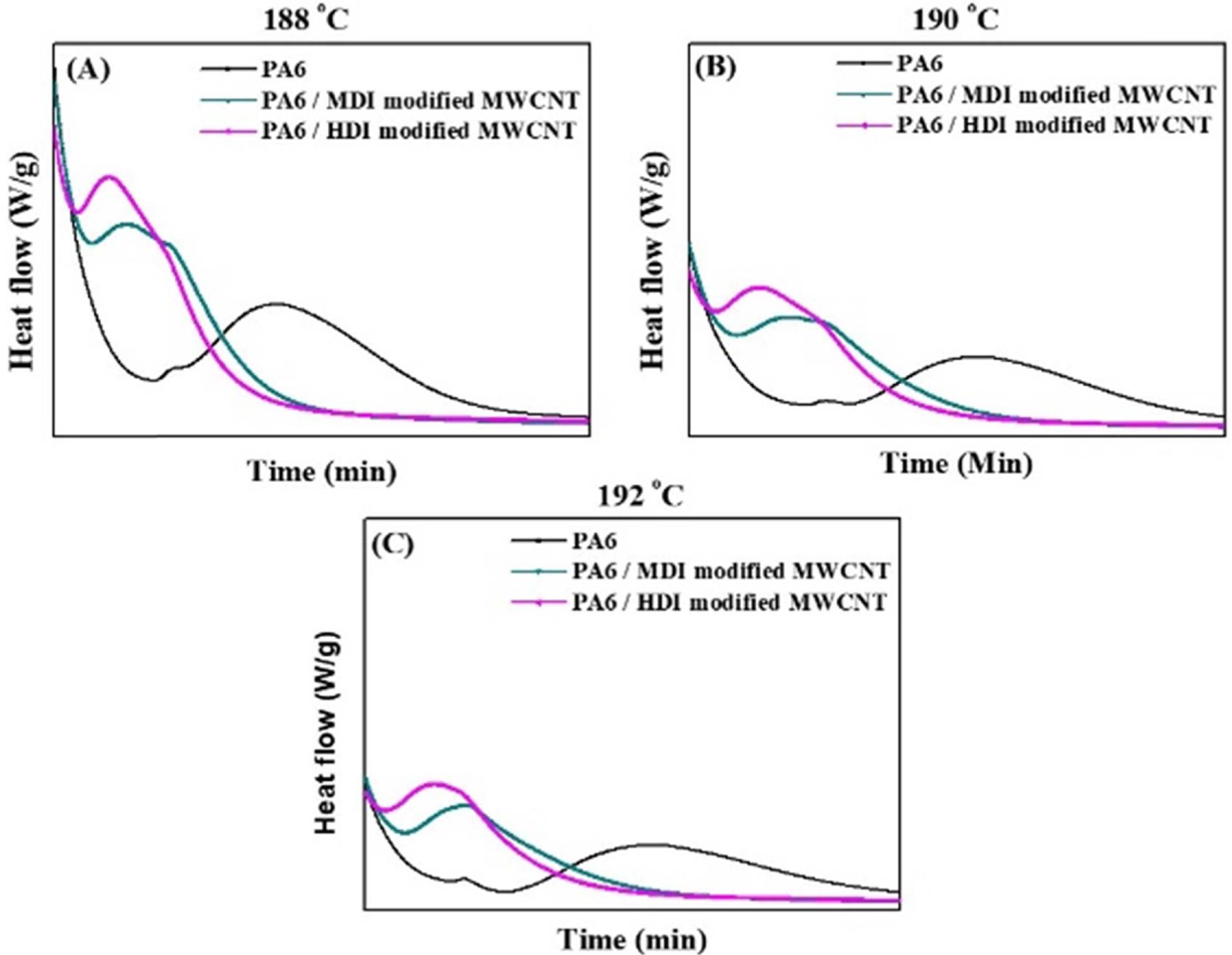
In
order to study the isothermal crystallization kinetics, the heat release during
the process was monitored. Fig. 6a-c shows the
characteristic isothermal crystallization exotherms of PA6 and PA6 nanocomposites containing surface
modified CNTs with CNT content of 0.1%. From the Fig. 6, the relative
degree of crystallinity, X(t) at time t, can be
established by

where the denominator is the total area under the isothermal
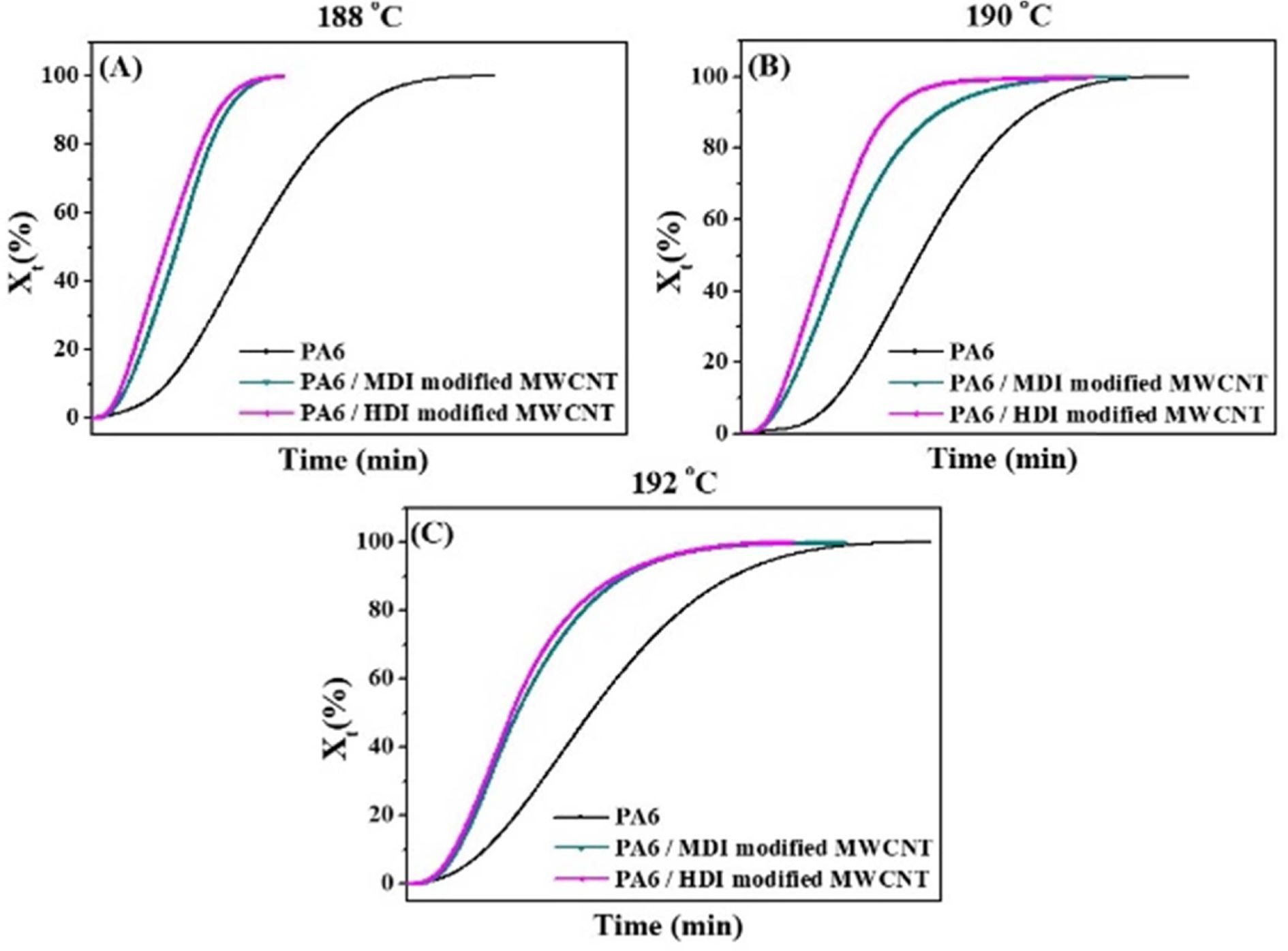
crystallization graph by DSC. Fig. 7 shows X(t) as a function of the
crystallization time t at different crystallization temperatures. A
typical sigmoidal evolution is seen in all three curves. From these Fig. 7, it can be
concluded that the addition of CNTs significantly shortened the corresponding
crystallization time of PA6. One of the most important rate parameter,
crystallization half-time (t0.5) which is defined as the time taken
the relative crystallinity of the sample reaches the value of 50% can be
directly obtained from Fig. 7.
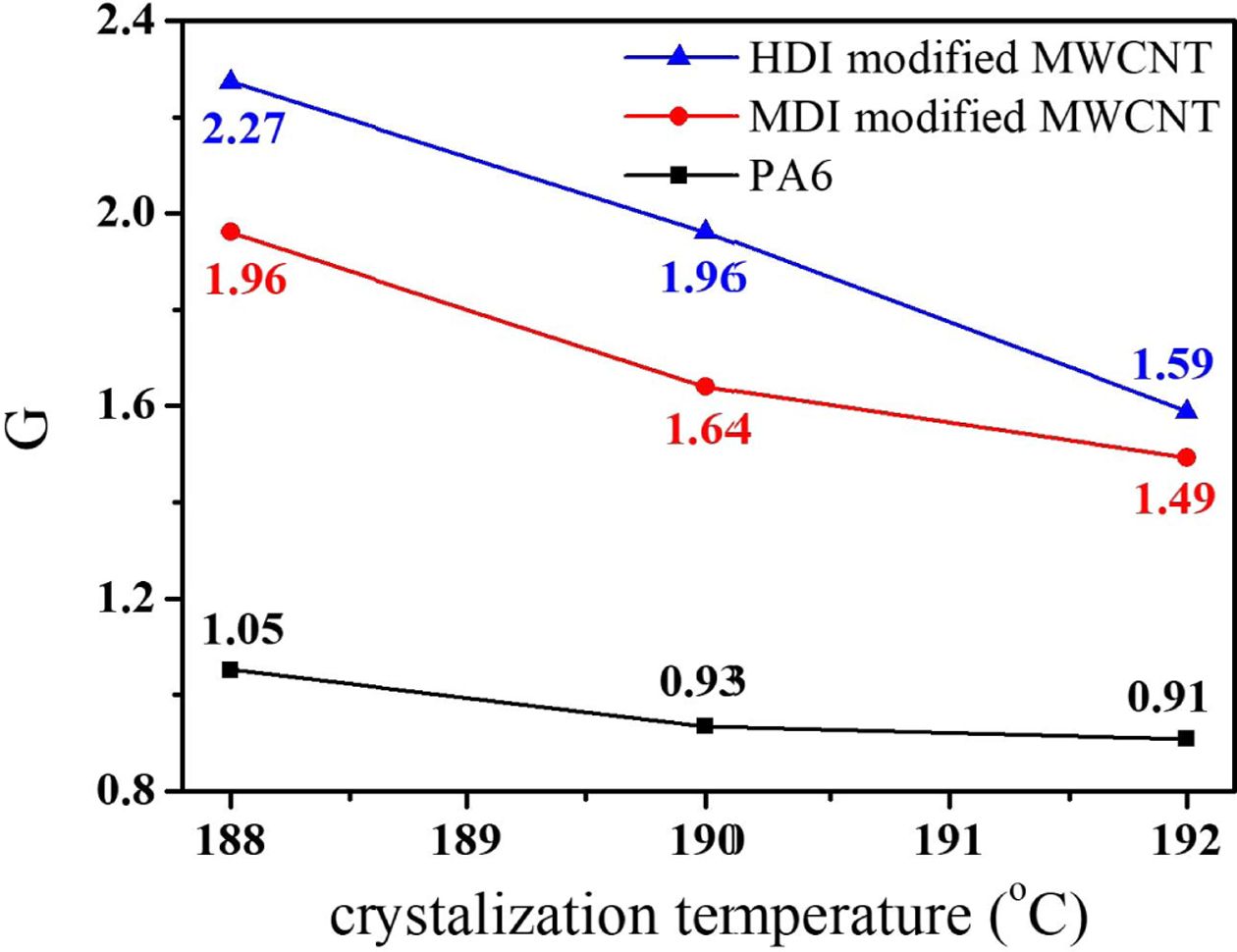
The
reciprocal (G) of t0.5 is usually used as the overall
crystallization rate and G values at different crystallization temperature of
188, 190, 192oC were summarized in Fig. 8. At all Tc,
the G value of the nanocomposites was greater than neat PA6 indicating that CNT
acted as a nucleating agent for PA6. Particularly, the HDI modified MWCNT
accelerated PA6 crystallization more efficiently than the MDI modified MWCNT.
The high surface PA6 density of CNTs may have resulted in stronger interfacial
interactions and compatibility of nanocomposite, than the low PA6 density of
CNT surfaces. The enhanced compatibility and interfacial interactions between
filler and matrices generally accelerates the nucleation of crystallization
very effectively. In addition, for all the samples the G value increased with Tc,
in agreement with the kinetics theory of crystallization, which expects for
crystallization close to the melting temperature, a decrease of undercooling
will slow the crystallization with increasing Tc. The isothermal
melt crystallization kinetics of the samples was further analyzed by the
well-known Avrami equation, which assumes the relative degree of crystallinity
(Xt) develops with crystallization time (t) as follows [18]:

or

where n is Avrami exponent which depends on the mechanism of nucleation
and growth of the crystal and k is the overall crystallization rate
constant. Plotting ln[-ln(1 - X(t)]
versus ln t therefore yields a straight line with a slope of n,
and the k value can be determined from the point of intersection with
the y axis.
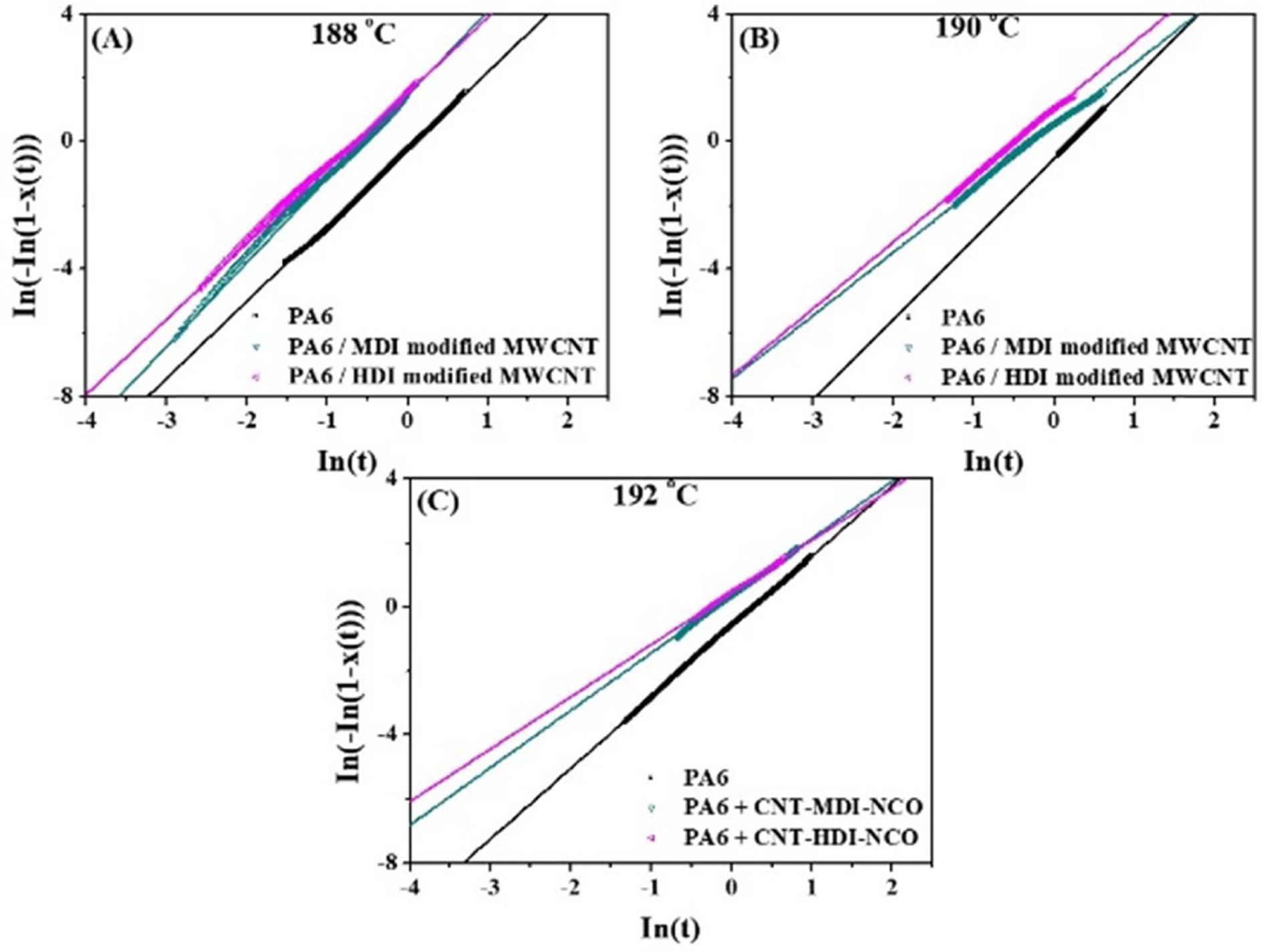
Fig.
9 shows the linear relation between ln[-ln(1 - X(t)] and ln t for the samples at
various Tc. Table 1 summarizes the values of n and k for
neat PA6 and its nanocomposites crystallized at different Tc. The k
values for the nanocomposites found to be greater than those for neat PA6 and
nanocomposites with HDI-modified CNTs which is consistent with G values. As
anticipated the k values also decreased with increasing Tc.
The Avrami exponent (n) represents the dimensionality of the growth,
three-dimensional crystallization a theoretical value of 3 should be obtained
for n. The lower-than-3 n values in the table could be due to mixed 3D
and 2D crystallization. For Tc = 188oC, the n
values were found to be 2.4~2.7 and almost unchanged with the addition of CNTs,
suggesting that despite the CNT loading, the crystallization mechanism of PA6
remained unchanged. However, at the higher Tc = 190 and 192oC,
n values decreased to 2.21 for PA6. Compared to PA6, the n values
were dropped further to 1.63 for nanocomposites with increasing Tc.
The changes in Avrami exponent after the addition of MWCNTs indicate that the
presence of MWCNT modified the growth pattern of PA6 crystals. With X(t)
= 0.5, from eq. (3) the relation
between the half-time and the crystallization parameter k can be
expressed as

The
half-time can be directly determined from X(t) data (Fig. 7) or be calculated
using eq. (4). Usually, the
Avrami equation can fit data in the primary crystallization range Nevertheless
a comparison between the experimental and the theoretically predicted values of
the half-crystallization time also indicates that the fits are very good up to
50% conversion since the values are quite similar.
|
Fig. 2 FTIR spectra of the MWCNT-OH, MDI and HDI-modified MWCNT |
|
Fig. 3 TGA data for the MWCNT-OH, MDI and HDI-modified MWCNT |
|
Fig. 4 The effect of MWCNTs on the degree of conversion |
|
Fig. 5 Effect of MWCNT loading on the mechanical properties of PA6/MWCNT nanocomposites, (a) the tensile strength and (b) Young’s modulus |
|
Fig. 6 DSC thermograms (cooling scan) of PA6 and PA6/MWCNT nanocomposites at various Tc at (a) 188 (b) 190 and (c) 192oC |
|
Fig. 7 Effect of surface modification on the relative degree of crystallinity, X(t) at different Tc |
|
Fig. 8 The variation of G value (=1/t0.5) as a function of Tc for PA6 and PA6/MWCNT nanocomposites |
|
Fig. 9 The linear relation of ln[-ln(1 - X(t)] and ln t for PA6 and PA6/MWCNT nanocomposites at various Tc at (a) 188 (b) 190 and (c) 192oC |
|
Table 1 Isothermal melt crystallization kinetic parameters for PA6 and MWCNT nanocomposites |
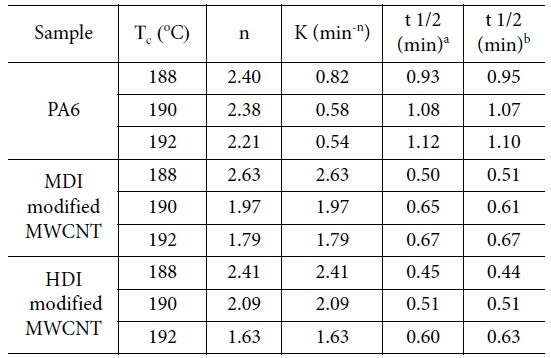
a Determined from Fig. 7 |
The
isocyanate functionalized MWCNTs was prepared by directly reacting commercial
hydroxyl functionalized MWCNTs with excess MDI and HDI. HDI-modified MWCNTs
resulted in the CNTs with higher isocyanate density than MDI-modified MWCNTs.
Anionic ring-opening polymerization of ECL was conducted by using a C10
initiator in combination with a C20 activator in the presence of isocyanate
functionalized CNTs as the second activator and reinforcing filler. This
polymerization proceeded in a highly efficient manner at relatively low
reaction temperature (150oC) and short reaction times (10 min). The
isocyanate functionalized CNTs improved nanocomposites more effectively than
hydroxyl functionalized CNTs. The results indicate that PA6 was successfully
grown from the MWCNT surface during the polymerization in the e-caprolactam melt. Nanocomposites with HDI modified MWCNTs
showed higher reinforcement and faster isothermal crystallization than MDI
modified MWCNTs. The results showed that PA6 chains grew more effectively from
HDI modified MWCNTs than from MDI modified MWCNTs, resulting in stronger
interaction between PA6 and MWCNT surface.
For
financial support of this research, we thank the core technology development
program (10052724) funded by the Ministry of Trade, Industry & Energy
(Republic of Korea).
- 1. Marchildon, K., “Polyamides - Still Strong After Seventy Years,” Macromolecular Reaction Engineering, Vol. 5, 2011, pp. 22-54.
-

- 2. Pervaiz, M., Faruq, M., Jawaid, M., and Sain, M., “Polyamides: Developments and Applications Towards Next-Generation Engineered Plastics,” Current Organic Synthesis, Vol. 14, 2017, pp. 146-155.
-

- 3. Winnacker, M., “Polyamides and Their Functionalization: Recent Concepts for Their Applications as Biomaterials,” Biomaterial Science, Vol. 5, 2017, pp. 1230-1235.
-

- 4. Feldman, D., “Polyamide Nanocomposites,” Journal of Macromolecular Science, Part A-Pure and Applied Chemistry, Vol. 54, 2017, pp. 255-262.
-

- 5. Faridirad, F., Ahmadi, S., and Barmar, M., “Polyamide/Carbon Nanoparticles Nanocomposites: A Review”, Polymer Engineering and Science, Vol. 57, 2017, pp. 475-494.
-

- 6. van Rijswijk, K., Teuwen, J.J.E., Bersee, H.E.N., and Beukers, A., “Textile Fiber-reinforced Anionic Polyamide-6 Composites. Part I: The Vacuum Infusion Process”, Composites Part A-Applied Science and Manufacturing, Vol. 40, 2009, pp. 1-10.
-

- 7. van Rijswijk, K., van Geenen, A.A., and Bersee, H.E.N., “Textile Fiber-reinforced Anionic Polyamide-6 Composites. Part II: Investigation on Interfacial Bond Formation by Short Beam Shear Test”, Composites Part A-Applied Science and Manufacturing. Vol. 40, 2009, pp. 1033-1043.
-

- 8. Barhoumi, N., Maazouz, A., Jaziri, M., and Abdelhedi, R., “Polyamide from Lactams by Reactive Rotational Molding via Anionic Ring-opening Polymerization; Optimization of Processing Parameters”, Express Polymer Letters, Vol. 7, 2013, pp. 76-87.
-

- 9. Vicard, C., De Almeida, O., Cantarel, A., and Bernhart, G., “Experimental Study of Polymerization and Crystallization Kinetics of Polyamide 6 Obtained by Anionic Ring Opening Polymerization of Epsilon-caprolactam”, Polymer, Vol. 132, 2017, pp. 88-97.
-

- 10. Maazouz, A., Lamnawar, K., and Dkier, M., “Chemorheological Study and In-situ Monitoring of PA6 Anionic-ring Polymerization for RTM Processing Control”, Composites Part A-Applied Science and Manufacturing, Vol. 107, 2018, pp. 235-247.
-

- 11. Ajayan P.M., and Tour, J.M., “Nanotube Composites”, Nature, Vol. 447, 2007, pp. 1066-1068.
-

- 12. Sahoo, N.G., Rana, S., Cho, J.W., Li, L., and Chan, S.H., “Polymer Nanocomposites Based on Functionalized Carbon Nanotubes”, Progress in Polymer Science, Vol. 35, 2010, pp. 837-867.
-

- 13. Spitalsky, Z., Tasis, D., Papagelis, K., and Galiotis, C., “Carbon Nanotube-polymer Composites: Chemistry, Processing, Mechanical and Electrical Properties”, Progress in Polymer Science Sci., Vol. 35, 2010, pp. 357-401.
-

- 14. Yang, M., Gao, Y., Li, H., and Adronov, A., “Functionalization of Multiwalled Carbon Nanotubes with Polyamide 6 by Anionic Ring-opening Polymerization”, Carbon, Vol. 45, 2007, pp. 2327-2333.
-

- 15. Yan, D., and Yang, G., “Synthesis and Properties of Homogeneously Dispersed Polyamide 6/MWNTs Nanocomposites via Simultaneous in situ Anionic Ring‐opening Polymerization and Compatibilization”, Journal of Applied Polymer Science, Vol. 112, 2009, pp. 3620-3626.
-

- 16. Oumi, L., Mirzaei, M., Ashtari, P., Ramazani, A., Rahimi, M., and Bolourinovin, F., “Isocyanate Functionalized Multiwalled Carbon Nanotubes for Separation of Lead from Cyclotron Production of thallium-201”, Journal of Radioanalytical and Nuclear Chemistry, Vol. 310, 2016, pp. 633-643.
-

- 17. Bernat, P., Hladka, O., Fismanova, M., Roda, J., and Brozek, J., “Polymerization of Lactams. 98: Influence of Water on Thenon-activated Polymerization -caprolactam”, European Polymer Journal, Vol. 44, 2008, pp. 32-41.
-

- 18. Avrami, M., “Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III”, The Journal of Chemical Physics, Vol. 9, 1941, pp. 177-183.
-

 This Article
This Article
-
2019; 32(6): 375-381
Published on Dec 31, 2019
- 10.7234/composres.2019.32.6.375
- Received on Oct 9, 2019
- Revised on Oct 31, 2019
- Accepted on Dec 9, 2019
 Services
Services
- Abstract
1. introduction
2. experimental
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seok Il Yun
-
Department of Chemical Engineering and Materials Science, Sangmyung University, Seoul 03016, Korea
- E-mail: yunsans@smu.ac.kr






 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.