- An Investigation of Interfacial Strength in Epoxy-based Solid Polymer Electrolytes for Structural Composite Batteries
Mohamad A. Raja*, Su Hyun Lim*, Doyun Jeon**, Hyunsoo Hong*, Inyeong Yang*, Sanha Kim*, Seong Su Kim*†
* Department of Mechanical Engineering, Korea Advanced Institute of Science and Technology (KAIST)
** Department of Aerospace Engineering, Korea Advanced Institute of Science and Technology (KAIST)This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Multifunctional composite materials capable of both load-carrying and energy functions are promising innovative candidates for the advancement of contemporary technologies owing to their relative feasibility, cost-effectiveness, and optimized performance. Carbon fiber (CF)-based structural batteries utilize the graphitic inherent structure to enable the employment of carbon fibers as electrodes, current collectors, and reinforcement, while the matrix system is an ion-conduction and load transfer medium. Although it is possible to enhance performance through the modification of constituents, there remains a need for a systematic design methodology scheme to streamline the commercialization of structural batteries. In this work, a bi-phasic epoxy-based ionic liquid (IL) modified structural battery electrolyte (SBE) was developed via thermally initiated phase separation. The polymer’s morphological, mechanical, and electrochemical characteristics were studied. In addition, the interfacial shear strength (IFSS) between CF/SBE was investigated via microdroplet tests. The results accentuated the significance of considering IFSS and matrix plasticity in designing composite structural batteries. This approach is expected to lay the foundation for realizing smart structures with optimized performance while minimizing the need for extensive trial and error, by paving the way for a streamlined computational design scheme in the future
Keywords: Structural Battery Electrolyte, Functional Smart Composites, Structural Battery, Interfacial Shear Strength, Epoxy-based Solid Electrolyte
Multifunctional energy systems, including structural batteries and supercapacitors, are promising solutions for advanced energy storage technologies and can be a valuable economical candidate for various industries such as automotive, aerospace, etc. [1,2]. The principle of materials’ multi-functionality is a key concept for developing high-performance structural batteries, whereby carbon fibers (CFs) serve as reinforcement, an active electrode, and a current collector, while the polymer matrix functions as ion-conduction and load-transfer media. However, the mechanical and electrochemical properties of the constituent materials, are inherently intertwined, creating a trade-off relationship [3,4]. In order to achieve a multifunctional composite material plausible for both simultaneous load-carrying and energy supply, enhancement of the constituents can be applied to the CF-based electrodes and/or the polymer matrix system.
CFs have been widely used as functional composites, primarily because of their exceptional mechanical properties. Additionally, the intrinsic graphitic structure grants high thermal stability and electrochemical characteristics. In a pioneering study from the U.S. Army Research Laboratory, Snyder et al. [5] analyzed the effect of pitch and polyacrylonitrile (PAN)-based fibers based on their mechanical performance, resistivity, and electrochemical capacity. The study concluded that PAN-based CFs have the greatest potential to be employed as multifunctional composites, as they demonstrated their ability to be used as an active anode in batteries supplanting the conventionally used graphite. However, most researchers tend to employ CFs as a skeleton for active material coatings, such as carbon nanotubes [6], graphite [7], or metal-based structures [8] for enhanced performance. Nano metal-modified CFs showed the greatest improvement as they exhibited electrochemical enhancement alongside increased mechanical performance due to the increased specific surface area between the CFs and the matrix system (i.e., increased adhesion). For instance, Cheng et al. [6] coated Co3O4 nanosheet arrays on carbon cloth by electrochemical deposition method, and the electrode showed superior electrochemical capacity of 787 mAh/g after 100 cycles at a current density of 0.4 mA/cm2. In addition, Huang et al. [9] synthesized sulfur and lithium electrodes by using carbon fabrics as the skeleton in an electrodeposition-like reaction, achieving 9.2 ± 1.2 GPa and 4.5 ± 0.6 GPa, respectively, 5–20 times higher than conventional electrodes showing better resistance to mechanical puncture alongside reaching an energy density of 43 Wh/kg with respect to the total mass of the system. These results and consistent research efforts show the promising potential of CFs as structural energy components, either by achieving a complete functionality of the CFs (i.e., electrodes, active materials, and current collectors) or by using their significant mechanical and morphological characteristics for the preparation of conformally-coated electrodes.
Liquid-based electrolytes have been widely adopted in commercial everyday batteries, as they offer high ionic conductivity, excellent wetting to the electrodes, and compatibility with mass production [10]. However, such solvents suffer from some drawbacks such as insufficient thermal stability, safety issues (i.e. leakage), volatility, and flammability. Consequently, many researchers are increasingly interested in polymer-based electrolyte systems; to allow the simultaneous multifunctional characteristics of load transfer, while enabling the exchange of lithium ions. Additionally, they exhibit advantages such as low flammability, easy processability, and resistance to mechanical deformations.
To this extent, polymer-based or solid-state electrolytes are generally based on polymer (e.g. poly(ethylene oxide) (PEO), poly(vinylidene fluoride) (PVDF), polyacrylonitrile (PAN), and poly(methyl methacrylate) (PMMA)) as the matrix and some lithium salts (e.g., LiClO4, LiTFSI (LiN(CF3SO2)2), LiAsF6, and LiPF6) [10,11]. Among the aforementioned systems, PEO is the most widely used in solid-state batteries [12]; allowing ions conduction through the amorphous region of the polymer chains where ions tend to hop from one coordinating site (i.e., electro-donor group) to another facilitated by the segmental polymer chain movement. Nevertheless, such systems have low ionic conductivity in the range of 10−8 to 10−4 S/cm compared to 10−3 to 10−2 S/cm of carbonate-based liquid electrolytes [12,13]. Furthermore, they have poor mechanical performance as Young’s Modulus of typical pristine PEO/LiTFSI is only 0.4 MPa. Consequently, due to the aforementioned aspects, epoxy-based polymer electrolytes have become increasingly appealing, further facilitating the use of structural composite batteries. However, the highly crosslinked crystalline chains pose a challenge as ion conduction cannot be facilitated by the chain’s mobility. Therefore, an ion-conduction medium through the epoxy domain is essential, however, the high temperatures used in processing high-performance epoxies restrict the usage of commercial electrolytes due to safety concerns.
Nevertheless, an ionic liquid (IL) based electrolyte can be a feasible solution for the realization of structural composite batteries as ILs have superior thermal stability, ionic conductivity, and compatibility with a wide range of polymers [14]. While CFs have high compatibility with the structural epoxy domain, the highly conductive IL phase in the polymer has been an efficient strategy in advancing the development of structural batteries reaching unprecedented capabilities [15,16].
However, such systems have an economical drawback as the cost of the ionic liquid is significantly higher reaching 2 to 100 times the cost of organic solvents [17]. Secondly, the effect of the fabricated bi-phasic polymer on the interfacial adhesion with the CFs has not been investigated thoroughly in previous research. Furthermore, the design of composite structural batteries has been relying on trial and error, lacking a systematic methodology with high certainty. Consequently, the aim of this work is to
1. Develop and characterize an epoxy-based IL-modified Structural Battery Electrolyte (SBE)
2. Study the effect of the SBE on the Interfacial Shear Strength (IFSS) with carbon fiber
3. Determine the Fiber Volume Fraction (FVF) of the carbon fiber’s cross-section via image processing
It is believed that this study can facilitate the global adoption of structural batteries by accentuating the significance of considering the SBE’s interfacial, and matrix plasticity in conjunction with their electrochemical capabilities during the design of structural composite batteries to diminish the reliance on trial and error and optimize performance.
2.1 Materials
Amine-cured Bisphenol A diglycidyl ether (DGEBA) epoxy was utilized as the structural phase in the polymer electrolyte system. For the liquid electrolyte phase, 1-Ethyl-3-methylimidazolium tetrafluoroborate (EMIMBF4; Sigma-Aldrich, Korea), propylene carbonate (PC; Sigma-Aldrich, Korea) were utilized as solvents for Lithium bis(trifluoromethanesulfonyl)imide (LiTFSI; MTI, Korea). The PAN-based CFs (H2550, Hyosung, Republic of Korea) were used in this study.
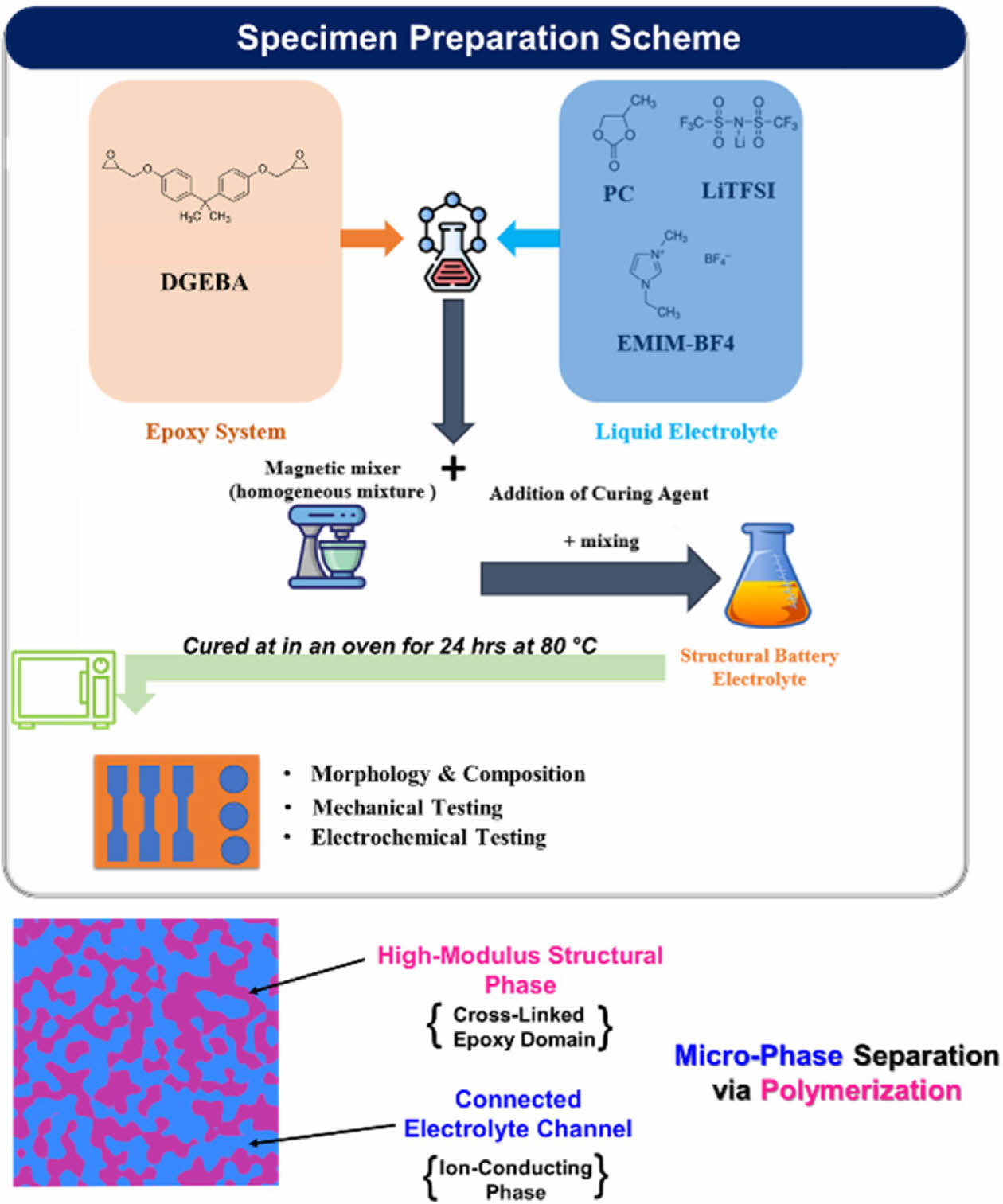
To prepare the polymer samples, 1 M LiTFSI was completely dissolved in 99 wt% of EMIMBF4 and 1% PC to formulate the liquid electrolyte phase, and then it was mixed with the epoxy phase using a magnetic stirring setup at room temperature until a homogeneous mixture was formed. The formulated polymer was mixed according to the mass ratio of the liquid electrolyte to epoxy, refereed as SBE_ (Electrolyte/Epoxy; i.e., SBE_0.75, SBE_1, SBE 1.25). Then it was cast in a steel mold to make specimens for further characterization. A schematic of the fabrication process with the resulting bi-phasic structures is shown in Fig. 1.
2.2 Scanning Electron Microscope (SEM)
SEM (AIS1800C, SERON, Korea) analysis was used to validate the bi-continuous polymer structure of SBEs, by observing the sample’s cross-section after extracting the electrolyte phase by ethanol immersion for around 2~3 Days. After the electrolyte extraction, the sample was dried overnight in an oven at 60 degrees to remove the ethanol, then it was quenched in liquid nitrogen and cut by a sharp edge knife to observe the cross-section.
2.3 Electrochemical Impedance Spectroscopy (EIS)
The ionic conductivity performance of the SBE specimens was evaluated by conducting an EIS analysis. The polymer sample was sandwiched between stainless steel electrodes with a thin film of conductive silver paste. The test was conducted in a frequency range of 0.01 Hz-1 MHz at a potential of 10 mV. The ionic conductivity was evaluated from the real axis resistance intersection and the sample’s geometry as illustrated in equation (1).

Where t is thickness, Rb is resistance, and A is area.
2.4 Mechanical Tensile Testing
To examine the polymer’s tensile properties dog-bone-shaped specimens were molded by a stainless mold. Fabricated specimens were tested according to ASTM D638 at a strain rate of 10 mm/min using a universal testing machine (UTM Instron 5969, Instron, USA), and the strain evolution was recorded by digital image correlation (DIC, GOM Correlate, Germany).
2.5 Interfacial Shear Strength (IFSS)
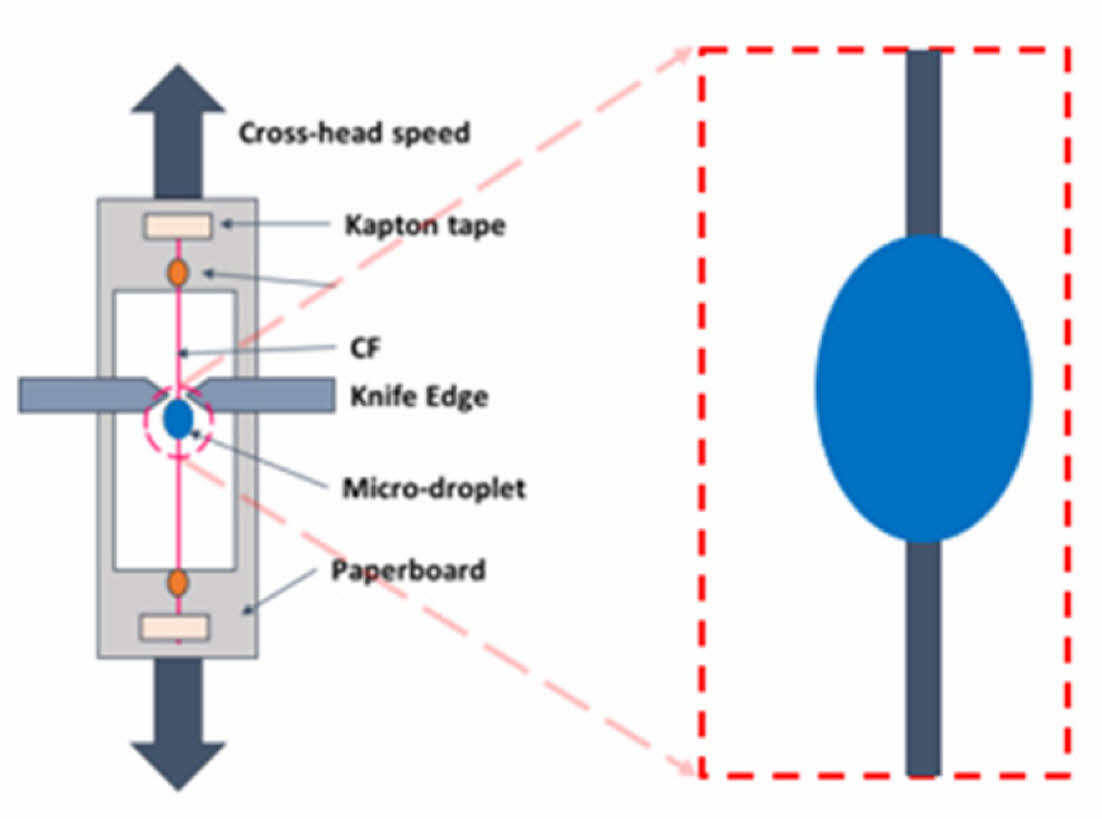
The IFSS between the CF’s surface and the SBE was evaluated by microdroplet test. A schematic of the specimen specification is shown in Fig. 2.
A microdroplet of the polymer [60~100 um] was placed on the carbon fiber surface by a needle tip and cured. The droplet was sheared in a mode II loading by a micrometer knife edge with a cross-head speed of 0.1 mm/min.
By recording the force-displacement curve, the IFSS can be evaluated according to equation (2)

where F is the max force, D is the fiber diameter, and L is the embedded length.
2.6 Fiber Volume Fraction (FVF)
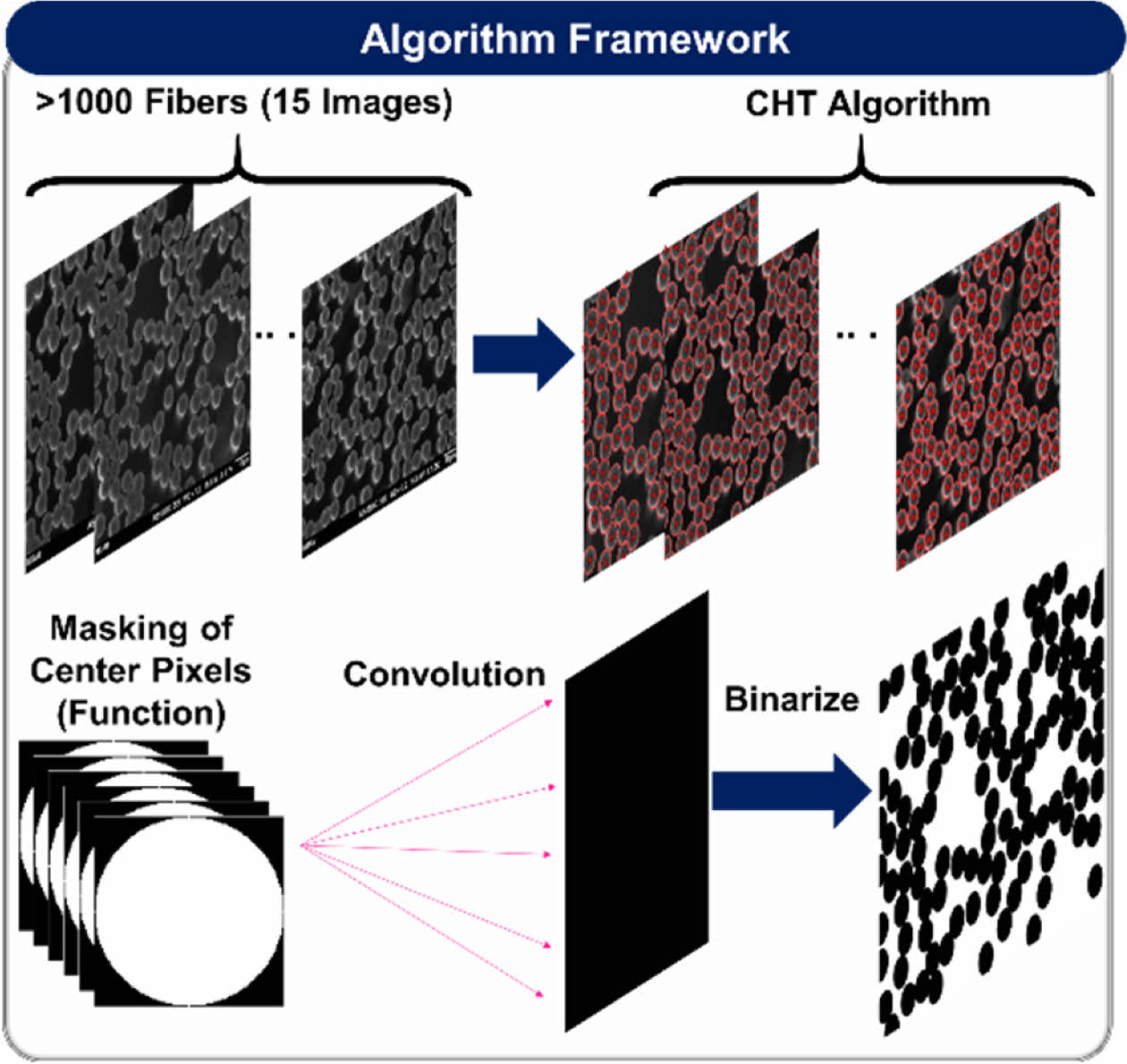
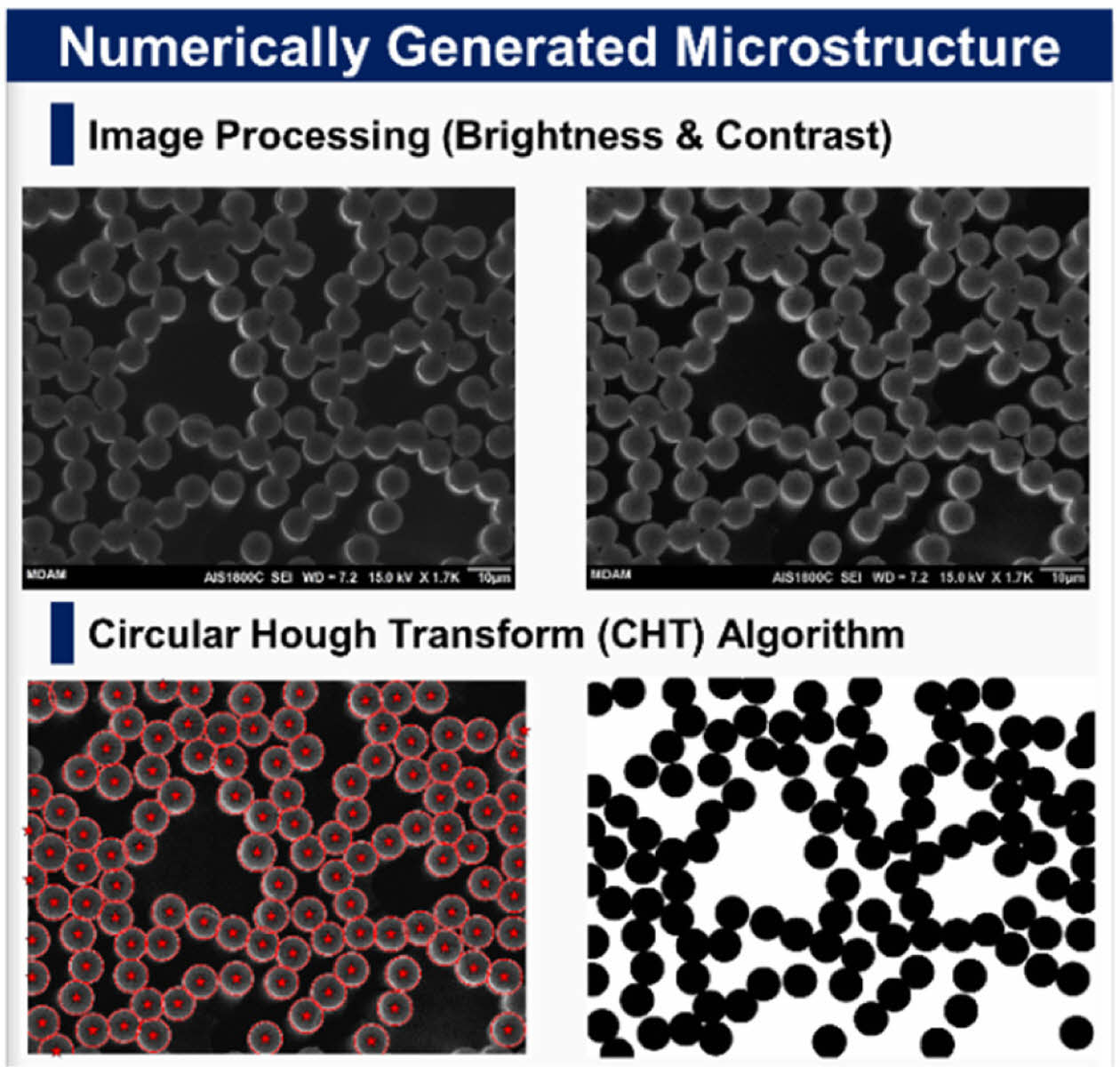
Prior to the computational analysis, the fiber volume fraction (FVF) must be identified to effectively study composite structural batteries as FVF is strongly related to both the mechanical and electrochemical performance. The FVF was analyzed according to ASTM D3171 (i.e., matrix burn) and cross-sectional image processing. The image processing was conducted using MATLAB in-house code to estimate the fibers centers and diameters via circular Hough transform (CHT) algorithm. Then the stored circles (i.e., centers and radii) were convoluted using an impulse function as shown in equation (3)
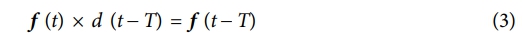
where; f: Function, d: Impulse Function, T: Shifting parameter, and *: Convolution. The overall algorithm framework is shown in Fig. 3.
|
Fig. 1 SBE preparation scheme & bi-phasic composition |
|
Fig. 2 IFSS specimen description. |
|
Fig. 3 Image Processing Algorithm Framework |
3.1 SBE Characterization
3.1.1 Morphology of SBE via SEM
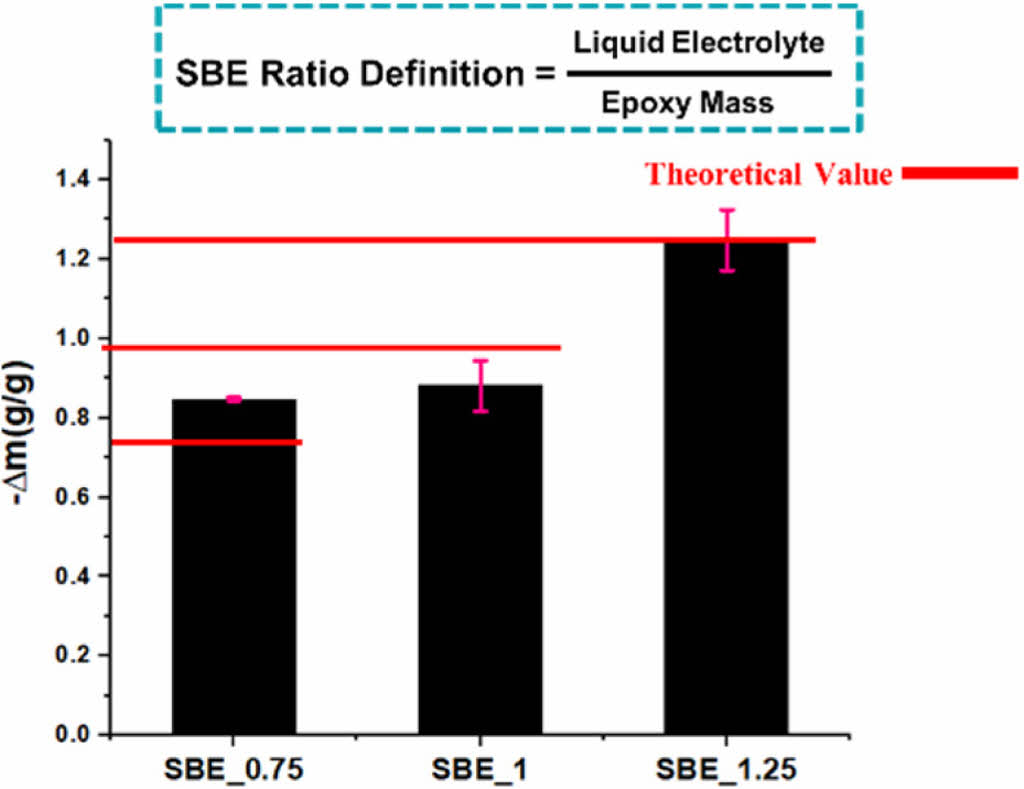
Fig. 4 shows the mass change percentage in the sample after the electrolyte extraction.
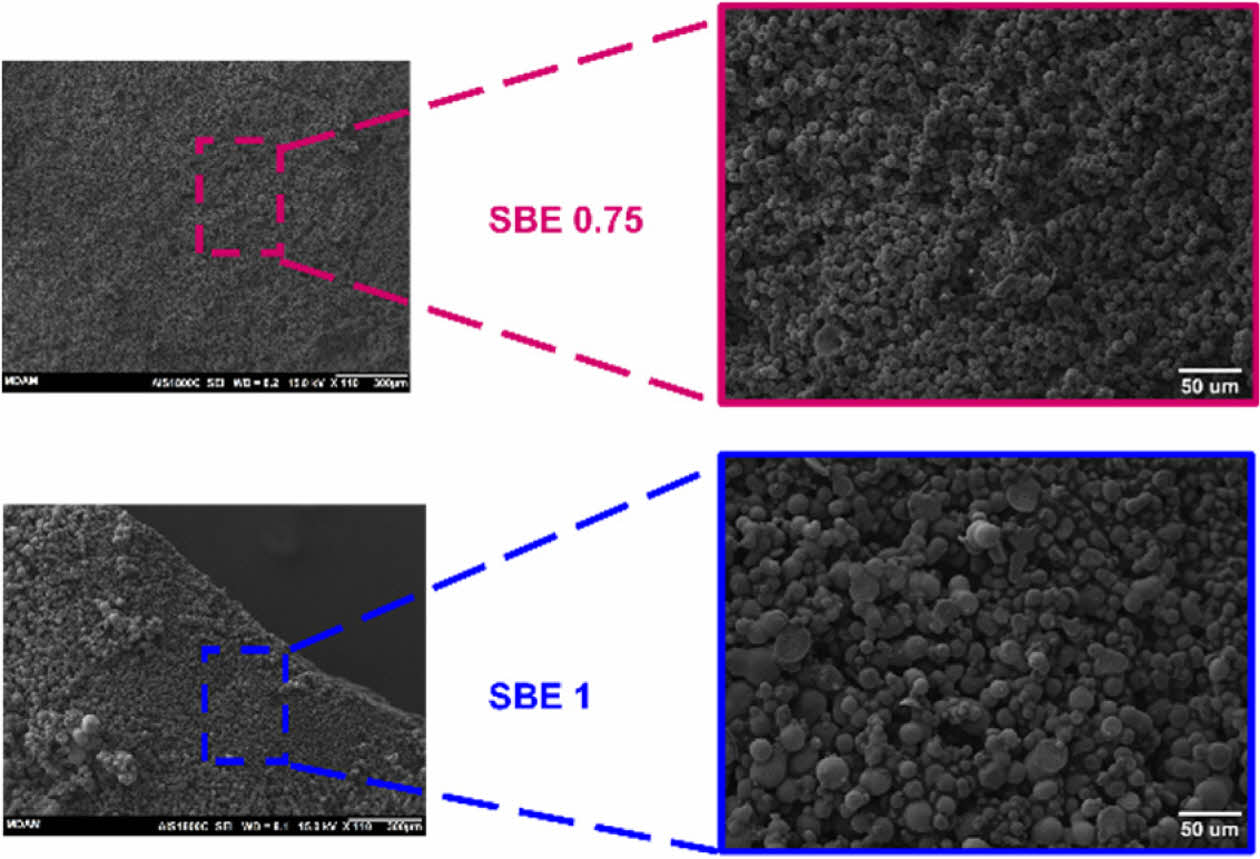
The similarity of mass change ∆m of the specimens indicates the even distribution in the epoxy-electrolyte mixtures. Fig. 5 shows the microstructure of two SBE samples.
SBE 1 shows a mixture of porous structure and node islands with various size distributions [e.g., harder for IL extraction due the epoxy network]. This can justify the lower extracted electrolyte mass in Fig. 4.
3.1.2 Evaluation of Mechanical/Electrochemical performance of SBE
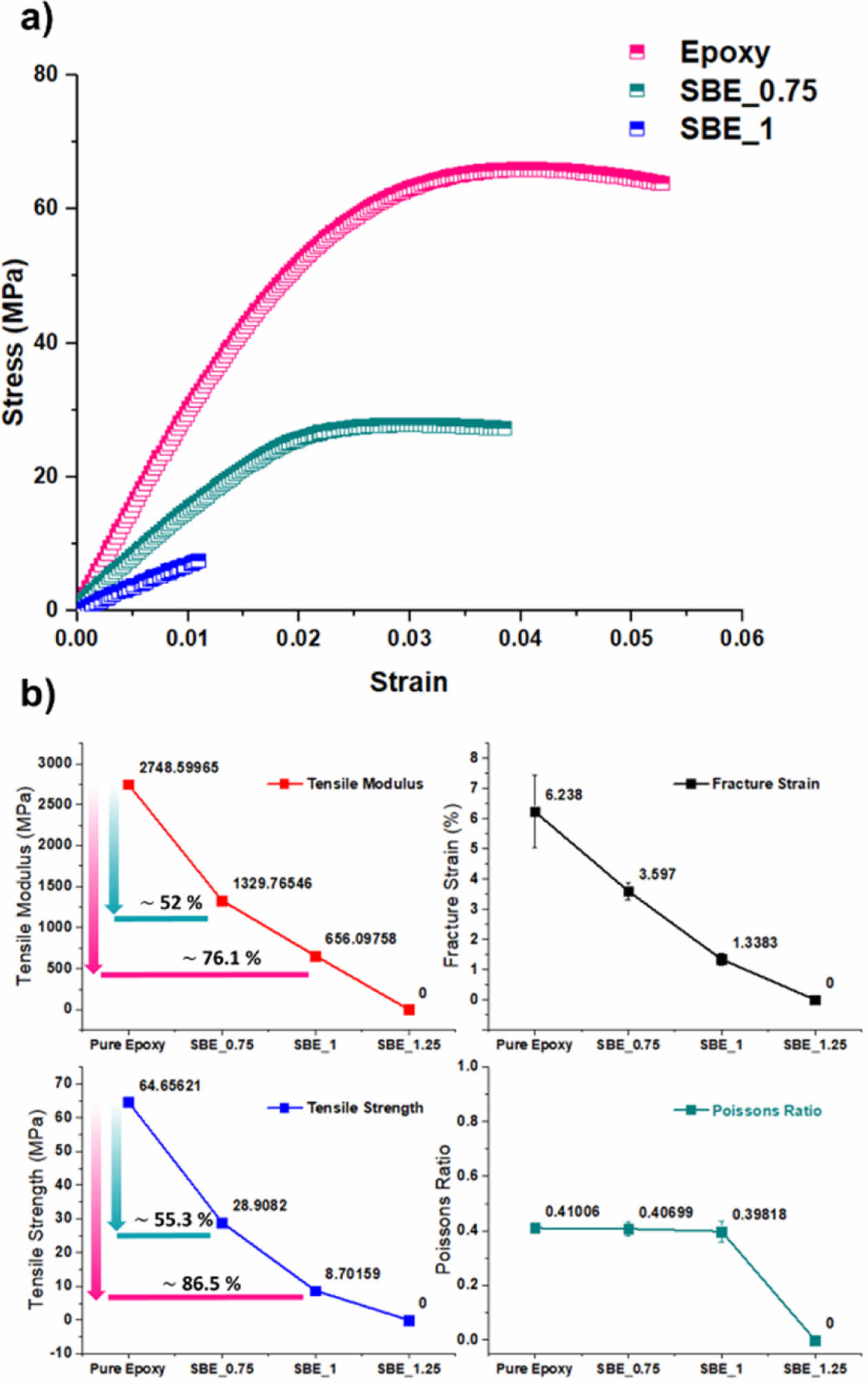
The stress-strain curves resulting from the tensile test are shown in Fig. 6a were the successive incorporation of the electrolyte decreases the ductile behavior of the polymer. The tensile modulus, strength, fracture strain, and Poisson’s ratio of the SBEs are shown in Fig. 6b.
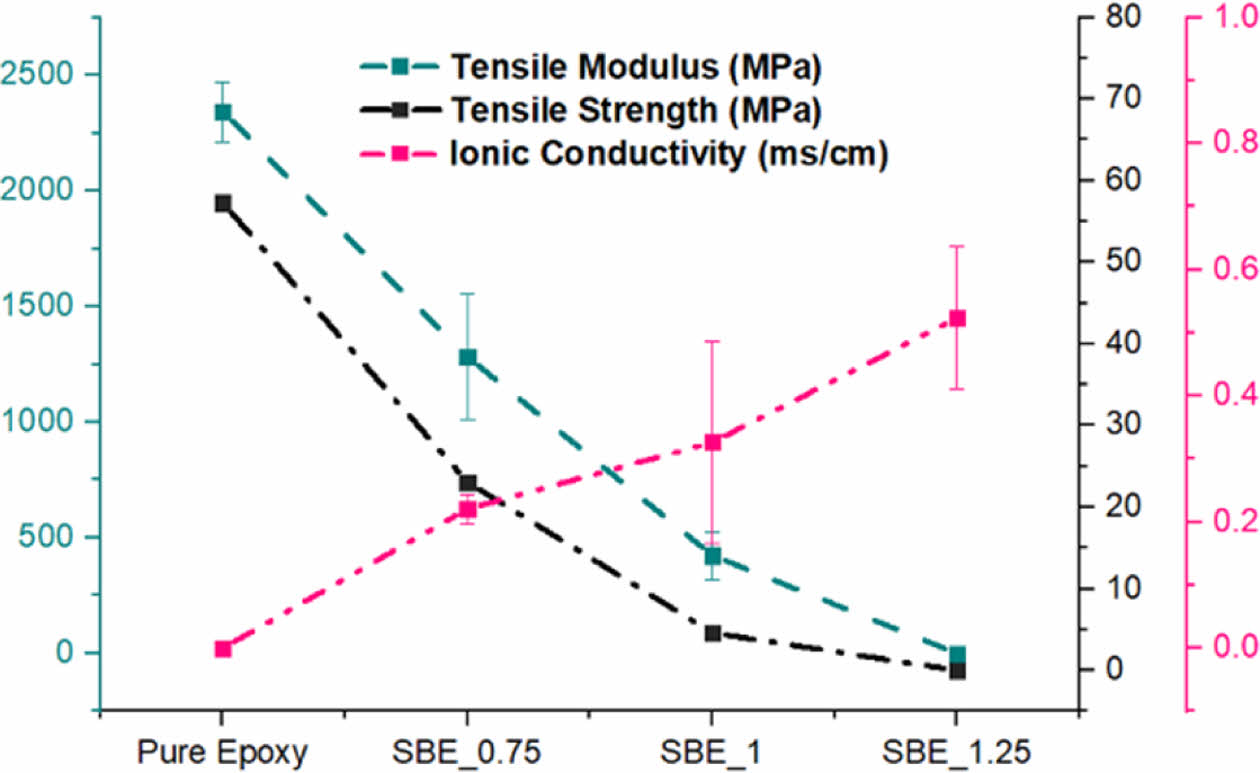
As it can be noticed, upon the introduction of electrolyte: the mechanical performance is significantly deteriorated. However, Poisson’s ratio is relatively constant, implying that although electrolyte is introduced, the axial and transverse strains are still proportionally related to the structural epoxy phase. Nevertheless, the degradation of the mechanical performance in the SBE is accompanied with a significant increase in electrochemical performance. As the increase of the electrolyte percentage, it promotes the continuous formation of the electrolyte channels within the epoxy phase. The trade-off relation can be seen in Fig. 7.
3.2 IFSS Experimental Test Result
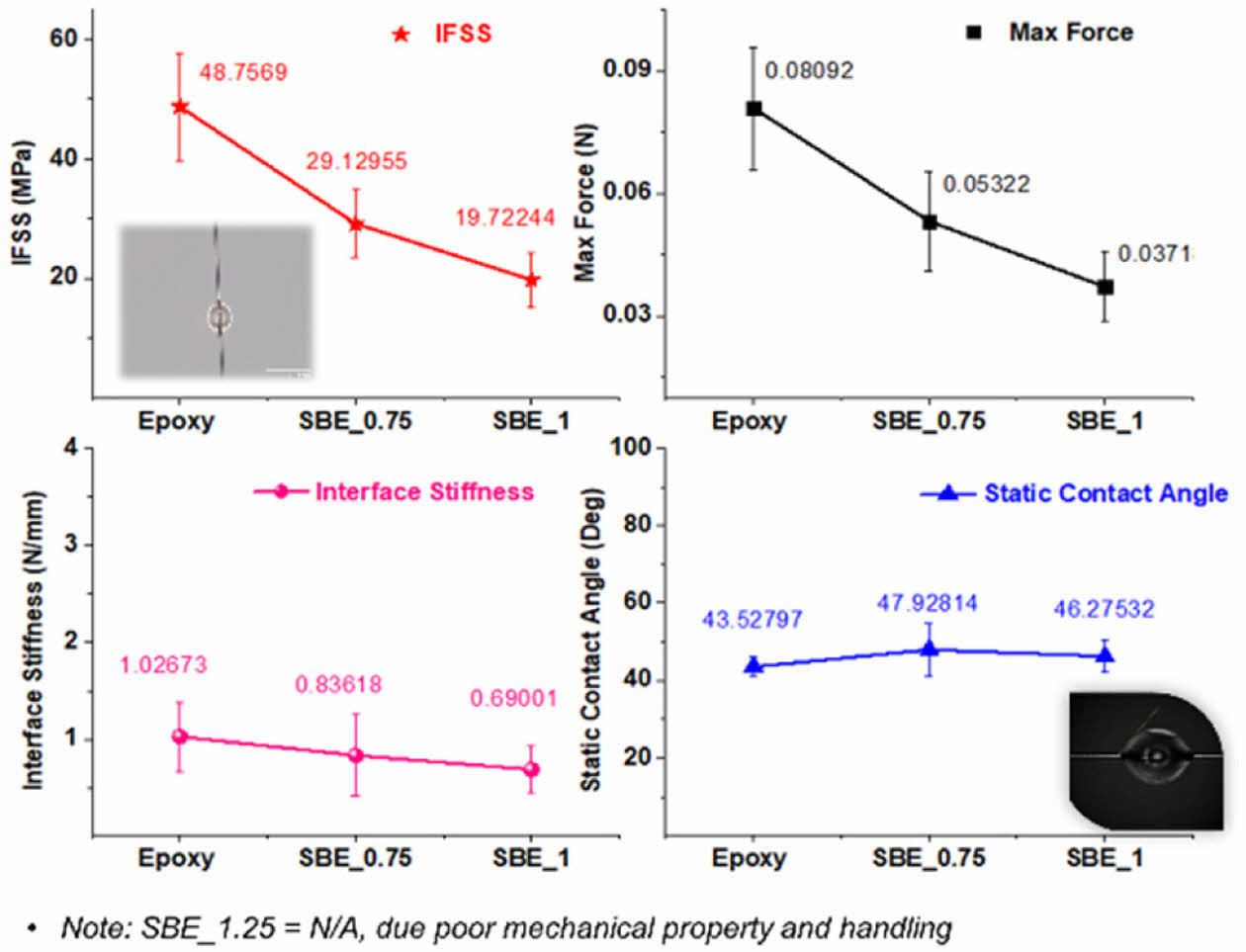
IFSS measurement results are shown in Fig. 8.
As it is expected due the lower mechanical performance (i.e. elastic modulus of the SBE) and the introduction of the electrolyte phase at the interface which does not take any load; there is a noticeable decrease in the IFSS as the electrolyte increases. Furthermore, the measurement of contact angle by single-fibers' droplets does not reveal any significant distinctions. Consequently, it can be deduced that the wettability of carbon fiber by the SBE was not notably altered in comparison to the epoxy scenario.
Overall, the results show that the interfacial strength deterioration is very critical and must be taking into consideration in the analysis and development of structural batteries, nevertheless, such analysis has not been considered in previous literature.
3.3 Fiber Volume Fraction (FVF) Measurement
The results for the FVF for the two methods, are shown in table (1), with around 5% difference. Also, image processing was proved to be a powerful technique to estimate the fiber diameter, and it was found to be 7.43 ± 0.4 um a very close approximation to the manufacturer’s reported diameter 7 um. An example of a processed binary image is shown in Fig. 9.
|
Fig. 4 Mass change percentage in the sample after the electrolyte extraction |
|
Fig. 5 Cross-sectional morphology of SBE via SEM |
|
Fig. 6 a) Stress-strain curves of different SBEs b) Polymer tensile test results |
|
Fig. 7 Trade-off relation of SBE's Mechanical/ Electrochemical performance |
|
Fig. 8 IFSS Experiment Results |
|
Fig. 9 Numerically Generated Microstructure of Fiber Crosssection for estimating FVF and Fiber Diameter |
Overall, in this study an epoxy-based/ IL SBE was developed via thermally initiated micro-phase separation. The microstructure, mechanical, and electrochemical properties were investigated. Also, the CF/SBE interface was analyzed via microdroplet interfacial shear strength.
The FVF, and fiber diameter were analyzed by image processing of more than 1000 fibers (> 15 images). The findings of the SBE’s multifunctional performance and FVF will be used to devise a framework for the design of structural batteries against interfacial and matrix plasticity, and application of CF based structural battery in a galvanostatic battery cell.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A2C201096512).
- 1. Kim, D.G., Koo, K.R., Kim, H.G., Song, S.C., Kwon, S.C., Lim, J.H., and Kim, Y.B., “Design and Analysis of Composite Reflector of High Stable Deployable Antenna for Satellite”, Composites Research, Vol. 36, No. 3, 2023, pp 230-240.
-

- 2. Asp, L.E., Johansson, M., Lindbergh, G., Xu, J., and Zenkert, D., “Structural Battery Composites: A Review,” Functional Composites and Structures, Vol. 1, No. 4, 2019.
-

- 3. Fu, Y., Zhou, H., and Zhou, L., “Phase-microstructure-mechanical Properties Relationship of Carbon Fiber Reinforced Ionic Liquid Epoxy Composites,” Composites Science and Technology, Vol. 207, 2021.
-

- 4. Kwon, S.J., Choi, U.H., Jung, B.M., Kim, Y.D., and Lee, S.B., “Effect of the Curing Behavior on Electrical and Mechanical Properties of Multifunctional Structural Electrolyte”, Composites Research, Vol. 29, 2016, pp 395-400.
-

- 5. Snyder, J.F., Wong, E.L., and Hubbard, C.W., “Evaluation of Commercially Available Carbon Fibers, Fabrics, and Papers for Potential Use in Multifunctional Energy Storage Applications,” Journal of The Electrochemical Society, Vol. 156, 2009.
-

- 6. Islam, M.S., Deng, Y., Tong, L., Faisal, S.N., Roy, A.K., Minett, A.I., and Gomes, V.G., “Grafting Carbon Nanotubes Directly onto Carbon Fibers for Superior Mechanical Stability: Towards Next Generation Aerospace Composites and Energy Storage Applications,” Carbon, Vol. 96, 2016, pp. 701-710.
-

- 7. Moyer, K., Meng, C., Marshall, B., Assal, O., Eaves, J., Perez, D., Karkkainen, R., Roberson, L., and Pint, C.L., “Carbon Fiber Reinforced Structural Lithium-ion Battery Composite: Multifunctional Power Integration for CubeSats,” Energy Storage Materials, Vol. 24, 2020, pp. 676-681.
-

- 8. Cheng, C., Zhou, G., Du, J., Zhang, H., Guo, D., Li, Q., Wei, W., and Chen, L., “Hierarchical Porous Co3O4 Nanosheet Arrays Directly Grown on Carbon Cloth by an Electrochemical Route for High Performance Li-ion Batteries,” New Journal of Chemistry, Vol. 38, No. 6, 2014, pp. 2250-2253.
-

- 9. Huang, W., Wang, P., Liao, X, Chen, Y., Borovilas, J., Jin, T., Li, A., Cheng, Q., Zhang, Y., Zhai, H., Chitu, A., Shan, Z., and Yang, Y., “Mechanically-robust Structural Lithium-sulfur Battery with High Energy Density,” Energy Storage Materials, Vol. 33, 2020, pp. 416-422.
-

- 10. Zheng, Y., Yao, Y., Ou, J., Li, M., Luo, D., Dou, H., Li, Z., Amine, K., Yu, A., and Chen, Z., “A Review of Composite Solid-state Electrolytes For Lithium Batteries: Fundamentals, Key Materials and Advanced Structures,” Chemical Society Reviews, Vol. 49, No. 23, 2020, pp. 8790-8839.
-

- 11. Zhou, D., Shanmukaraj, D., Tkacheva, A., Armand, M., and Wang, G., “Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects,” Chem, Vol. 5, No. 9, 2019, pp. 2326-2352.
-

- 12. Xue, Z., He, D., and Xie, X., “Poly (ethylene oxide)-based Electrolytes for Lithium-ion Batteries,” Journal of Materials Chemistry A, Vol. 3, No. 38, 2015, pp. 19218-19253.
-

- 13. Zhang, Y., Zheng, Z., Liu, X., Chi, M., and Wang, Y., “Fundamental Relationship of Microstructure and Ionic Conductivity of Amorphous LLTO as Solid Electrolyte Material,” Journal of The Electrochemical Society, Vol. 166, No. 4, pp. A515-A520, 2019.
-

- 14. Polu, A.R., and Rhee, H.W., “Ionic Liquid Doped PEO-based Solid Polymer Electrolytes for Lithium-ion Polymer Batteries,” International Journal of Hydrogen Energy, Vol. 42, No. 10, 2017, pp. 7212-7219.
-

- 15. Huang, F., Zhou, Y., Sha, Z., Peng, S., Chang, W., Cheng, X., Zhang, J., Brown, S.A., Han, Z., and Wang, C.H., “Surface Functionalization of Electrodes and Synthesis of Dual-Phase Solid Electrolytes for Structural Supercapacitors,” ACS Applied Materials & Interfaces, Vol. 14, No. 27, 2022, pp. 30857-30871.
-

- 16. Kwon, S.J., Kim, T., Jung, B.M., Lee, S.B., and Choi, U.H., “Multifunctional Epoxy-Based Solid Polymer Electrolytes for Solid-State Supercapacitors,” ACS Applied Materials & Interfaces, Vol. 10, No. 41, 2018, pp. 35108-35117.
-

- 17. Sowmiah, S., Srinivasadesikan, V., Tseng, M.C., and Chu, Y.H., “On the Chemical Stabilities of Ionic Liquids,” Molecules, Vol. 14, No. 9, 2009, pp. 3780-3813.
-

 This Article
This Article
-
2023; 36(6): 416-421
Published on Dec 31, 2023
- 10.7234/composres.2023.36.6.416
- Received on Oct 23, 2023
- Accepted on Nov 7, 2023
 Services
Services
- Abstract
1. introduction
2. materials & methods
3. results & discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Seong Su Kim
-
Department of Mechanical Engineering, Korea Advanced Institute of Science and Technology (KAIST)
- E-mail: seongsukim@kaist.ac.kr







 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.